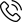Congratulations to Dr. Estrada for her feature in Business Wire on her scientific work in melanoma testing. Read more about the recent study on the future of skin cancer risks in patients.
FRIENDSWOOD, Texas–(BUSINESS WIRE)–Castle Biosciences, Inc., the skin cancer diagnostics company providing molecular diagnostics to improve cancer management decisions, today announced study results showing that the DecisionDx-Melanoma test accurately predicts risk for patients with cutaneous melanoma when using either biopsy or wide local excision (WLE) as the tissue source. Highlights of the study were presented during an oral presentation at the American Society of Dermatopathology 55thAnnual Meeting held November 8-11, 2018 in Chicago.
“Using biopsy or wide local excision tissue does not impact the ability of the DecisionDx-Melanoma test to accurately predict risk and inform patient management decisions.”
“This study demonstrates that the DecisionDx-Melanoma test provides an accurate, independent prediction of risk regardless of tissue source,” said Sarah Estrada, M.D., FACP, Affiliated Dermatology, Scottsdale AZ, a study investigator. “Using biopsy or wide local excision tissue does not impact the ability of the DecisionDx-Melanoma test to accurately predict risk and inform patient management decisions.”
Study Background
- The DecisionDx-Melanoma test has been shown to provide an accurate, independent prediction of risk for patients with cutaneous melanoma.
- The test has shown robust analytic reliability with 99% inter-assay concordance and 100% intra-assay concordance. Technical success exceeded 98% on samples with sufficient tumor content.
- This study assessed whether test results or patient outcomes were associated with tissue source (biopsy or WLE) in an archival cohort of 537 patients with Stage I-III cutaneous melanoma for whom the tissue source was known.
Key Study Results
- Among those who had biopsy tissue tested, a higher percentage had Stage I disease (68%) compared to those who had WLE tissue tested (19% Stage I; p<0.001).
- Patients who had WLE tissue tested were significantly more likely to have Stage III melanoma (50%) compared to those who had biopsy tissue tested (17% Stage III; p<0.001).
- Patients who had WLE tissue tested also were more likely to have higher risk pathologic features such as ulceration and increased Breslow thickness.
- For both tissue sources, patients who had the lowest risk DecisionDx-Melanoma test result (Class 1A) had significantly improved distant metastasis-free survival and melanoma-specific survival rates compared to those who had the highest risk test result (Class 2B).
- After adjusting for pathologic features among patients in the two tissue source groups, use of WLE tissue was not significantly associated with DecisionDx-Melanoma test class result.
- In multivariate analysis, DecisionDx-Melanoma Class 2B (highest risk) was a significant independent predictor of recurrence, distant metastasis and melanoma-specific survival, while WLE tissue source was not found to be predictive of outcomes.
- These results demonstrate that the DecisionDx-Melanoma test provides an accurate, independent prediction of risk regardless of tissue source.
About DecisionDx-Melanoma
The DecisionDx-Melanoma test uses tumor biology to predict individual risk of melanoma recurrence and sentinel lymph node positivity independent of traditional factors. Using tissue from the primary melanoma, the test measures the expression of 31 genes. The test has been validated in three multi-center studies that have included 690 patients and have demonstrated consistent results. Performance has also been confirmed in four prospective studies including 702 patients. The consistent high performance and accuracy demonstrated in these studies, which combined have included over 1,300 patients, provides confidence in disease management plans that incorporate DecisionDx-Melanoma test results.
Prediction of the likelihood of sentinel lymph node positivity has also been validated in two prospective multicenter studies that included over 1,400 patients. Impact on patient management plans for one of every two patients tested has been demonstrated in multi-center and single-center studies. More information about the test and disease can be found at www.SkinMelanoma.com.
About Castle Biosciences
Castle Biosciences is a molecular diagnostics company dedicated to helping patients and their physicians make the best possible decisions about their treatment and follow up care based on the individual molecular signature of their tumor. The Company currently offers tests for patients with cutaneous melanoma (DecisionDx®-Melanoma, DecisionDx®-CMSeq; www.SkinMelanoma.com) and uveal melanoma (DecisionDx®-UM, DecisionDx®-PRAME and DecisionDx®-UMSeq; www.MyUvealMelanoma.com), with development programs in other underserved cancers, the most advanced of which is focused on patients with cutaneous squamous cell carcinoma. Castle Biosciences is based in Friendswood, Texas (Houston), and has laboratory operations in Phoenix, Arizona. More information can be found at www.CastleBiosciences.com.
DecisionDx-Melanoma, DecisionDx-CMSeq, DecisionDx-UM, DecisionDx-PRAME and DecisionDx-UMSeq are the trademarks of Castle Biosciences, Inc. Any other trademarks are the property of their respective owners.
Contacts
Castle Biosciences, Inc.
Derek Maetzold, 866-788-9007
President and CEO
IR@castlebiosciences.com